Amitriptyline Hydrochloride
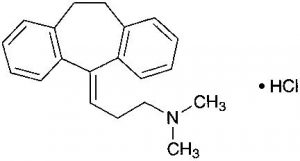
Amitriptyline Hydrochloride C20H23N.HCl: 313.86
3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethylpropylamine monohydrochloride [549-18-8]
Amitriptyline Hydrochloride, when dried, contains not less than 99.0% of amitriptyline hydrochloride (C20H23N.HCl).
Description
Amitriptyline Hydrochloride occurs as colorless crystals or a white to pale yellow crystalline powder. It has a bitter taste and a numbing effect.
It is freely soluble in water, in ethanol (95) and in acetic acid (100), soluble in acetic anhydride, and practically insoluble in diethyl ether.
The pH of a solution of 1.0 g of Amitriptyline Hydrochloride in 20 mL of water is between 4.0 and 5.0.
Identification
(1) Dissolve 5 mg of Amitriptyline hydrochloride in 3 mL of sulfuric acid: a red color develops. Add 5 drops of potassium dichromate TS to this solution: it turns dark brown.
(2) Acidify 1 mL of a solution of Amitriptyline hydrochloride (1 in 500) with 0.5 mL of dilute nitric acid, and add 1 drop of silver nitrate TS: a white, opalescent precipitate is produced.
(3) Determine the absorption spectrum of a solution of Amitriptyline hydrochloride (1 in 100,000) as directed under Ultraviolet-visible Spectrophotometry <2.24>, and compare the spectrum with the Reference Spectrum or the spectrum of a solution of Amitriptyline hydrochloride RS prepared in the same manner as the sample solution: both spectra exhibit similar intensities of absorption at the same wavelengths.
Melting point <2.60>
195 – 198oC
Purity
(1) Clarity and color of solution—Dissolve 1.0 g of Amitriptyline Hydrochloride in 20 mL of water: the solution is clear and colorless.
(2) Heavy metals <1.07>—Proceed with 2.0 g of Amitriptyline Hydrochloride according to Method 2, and perform the test. Prepare the control solution with 2.0 mL of Standard Lead Solution (not more than 10 ppm).
Loss on drying <2.41>
Not more than 0.5% (1 g, 105oC, 2 hours).
Residue on ignition <2.44>
Not more than 0.1% (1 g).
Assay
Weigh accurately about 0.5 g of Amitriptyline hydrochloride, previously dried, dissolve in 50 mL of a mixture of acetic anhydride and acetic acid (100) (7:3), and titrate <2.50> with 0.1 mol/L perchloric acid VS (potentiometric titration). Perform a blank determination, and make any necessary correction.
Each mL of 0.1 mol/L perchloric acid VS = 31.39 mg of C20H23N.HCl
Containers and storage
Containers—Tight containers.
Storage—Light-resistant.
THE JAPANESE PHARMACOPOEIA
SEVENTEENTH EDITION
PLEASE TO REFER VNRAS.COM



































