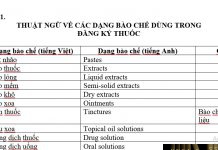
List of APIs stopped granting registration number circulating in Vietnam
| SN | APIs | REGULATIONS | CONTENT | APPLIED TIME |
| 1 | Phenylbutazone | The official letter 2706/QLD | Stop granting new, renew registration numbers. Stop importing material, finished product containing Phenylbutazone. Stop manufacturing product containing Phenylbutazone granted registration numbers. | 14-May-2001 |
| 2 | Philatop | Undefined | Stop receiving new, renew dossiers | Undefined |
| 3 | Opi extract | Undefined | Stop receiving new, renew dossiers | Undefined |
| 4 | Chloramphenicol (Material powder packed in sachets) | Undefined | Stop receiving new, renew dossiers | Undefined |
| 5 | Spartein sulfat | Undefined | Stop receiving new, renew dossiers | Undefined |
| 6 | Vitamin A > 5.000 UI/tablet or > 2.500 UI/ml | Undefined | Stop receiving new, renew dossiers | Undefined |
| 7 | Phenobarbital in combination with Paracetamol | Undefined | Stop receiving new, renew dossiers | Undefined |
| 8 | Nimesulid | The official letter 341/QLD-DK | Stop receiving new, renew dossiers | 9-Jan-2008 |
| The official letter 3790/QLD-ĐK | Continuing receving dossiers for external adminstrition | 5-May-2008 | ||
| 9 | Sibutramin | The official letter 5678/QLD-ĐK | Stop receiving new, renew dossiers | 8-Jun-2010 |
| 10 | Meprobamate pure form or combination with Valerian | The official letter 5865/QLD-ĐK | Stop receiving new, renew dossiers | 24-Apr-2012 |
| 11 | Aceprometazine pure form or combination with Clorazepate | Undefined | Stop receiving new, renew dossiers | Undefined |
| 12 | Combined drug containing Acid acridon and N-Methylglucamin | Undefined | Stop receiving new, renew dossiers | Undefined |
| 13 | Serratiopeptidase | The official letter 5868/QLD-ĐK | Stop importing, registration | 24-Apr-2012 |
| 14 | Ketoconazole oral rute | The official letter 5869/QLD-ĐK | Stop receiving new, renew dossiers | 24-Apr-2012 |
| 15 | Combined drugs containing APIs Streptokinase and Streptodornase | The official letter 13702/QLD-ĐK | Stop receiving imported licenses for combined drugs, and materials for purposes of producing above combinations | 13-Sep-2012 |
| The official letter 17499/QLD-KD | Stop importing | 13-Nov-2012 | ||
| 16 | Combination Cefixim/Acid clavunanic; Ceftriazone/Sulbactam or similar combination between Cephalosporin and Beta-lactamase inhibitors (except Cefoperazone/Sulbactam) | The official letter 13703/QLD-ĐK | Stop receiving imported licenses for combined drugs, and materials for purposes of producing above combinations | 13-Sep-2012 |
| The official letter 17499/QLD-KD | Stop importing | 13-Nov-2012 | ||
| 17 | Sodium hydrocarbonate pure form for oral administration with indication of antacid | Undefined | Stop granting new, renew registration numbers | Undefined |
| 18 | Activated alpha drotrecogin | Undefined | Stop granting new, renew registration numbers | Undefined |
| 19 | Duxil (Almitrin-Raubasin) | The official letter 4398/QLD-ĐK | Stop granting new, renew registration numbers | 9-Apr-2012 |
| The official letter 18428/QLD-ĐK | ||||
| 20 | Pioglitazone | The official letter 13707/QLD-ĐK | Stop granting new, renew registration numbers | 13-Sep-2012 |
| 21 | Rosiglitazon | The official letter 3886/QLD-ĐK | Stop importing, registration | |
| 22 | Buflomedil | The official letter 12792/QLD-CL | Suspend circulation and recall nationwide all products | 27-Aug-2012 |
| The official letter 13706/QLD-ĐK | Stop importing, registration | 13-Sep-2012 | ||
| 23 | Medicines containing herbal component “thạch xương bồ” | The official letter 14975/QLD-ĐK | Stop granting new, renew registration numbers | 2-Oct-2012 |
| 24 | Medicines containing herbal component “biển súc” | Undefined | Request providing clinical documents | Undefined |
| 25 | Oral administration drugs containing Artemisinin or derivatives of Artemisinin | The decision 112/QĐ-QLD | Withdraw registration numbers | 20-May-2013 |
| The official letter 7873/QLD-CL | Stop using and recall nationwide | 21-May-2013 | ||
| 26 | BDD (biphenyl dimethyl dicarboxylat) or Bifendat | The official letter 4822/QLD-ĐK | Stop granting new, renew registration numbers. For valid registration numbers requested providing clinical documents. | 31-Mar-2014 |
| 27 | Cefetamet | The official letter 4810/QLD-ĐK | Stop granting new, renew registration numbers. For valid registration numbers requested providing clinical documents. | 28-Mar-2014 |
| 28 | Combined medicine containing Vitamins and minerals for oral aministration | The official letter 16751/QLD-ĐK | Require to provide clinical studies for the combination drugs without providing origin of formula based on formal references or formulas, constituent granted registration numbers for circulation by references authories | 29-Sep-2014 |
| 29 | Metoclopramid | The official letter 16752/QLD-ĐK | Stop receiving new, renew registration dossiers, and do not grant registration numbers for drugs containing metoclopramide with dosage forms and routes of administration as recommended by the EMA, in particular: – The liquid medicine for oral in children have larger concentrations of 1 mg/ml – Preparations for intravenous have larger concentration of 5mg/ml – Rectal administration preparations doses of 20mg. | 29-Sep-2014 |
| 30 | Gatifloxacin | The decision 101/QD-QLD | Withdraw registration numbers | 14-Feb-2015 |
| 31 | Tetrazepam | The official letter 5078/QLD-ĐK | Stop importing, registration, circulation | 20-Mar-2015 |
| 32 | Lysozym | The official letter 1209/QLD-CL | Suspend circulation and recall nationwide all products containing Lysozym | 23-Jan-2015 |
| The official letter 5131/QLD_CL | Suspend circulation and recall nationwide all products containing Lysozym | 23-Mar-2015 | ||
| 33 | Biphenyl dimethyl dicarboxylat, Cefetamet | The decision 442/QD-QLD | Withdraw registration numbers, suspend circulation, recall nationwide all nationwideproducts containing APIs Biphenyl dimethyl dicarboxylat, Cefetamet | 10-Aug-2015 |
DOWNLOAD LIST OF APIS HERE
[sociallocker id=7424]
Checklist APIs stopped granting registration numbers
[/sociallocker]
DANH SÁCH HOẠT CHẤT NGỪNG CẤP SỐ ĐĂNG KÝ LƯU HÀNH TẠI VIỆT NAM TẠI ĐÂY
CẬP NHẬT, CẢNH BÁO THÔNG TIN HOẠT CHẤT ĐANG LƯU HÀNH TẠI VIỆT NAM
COPY VUI LÒNG GHI NGUỒN VNRAS.COM